U.S. FDA advisors back bivalent COVID vaccine for initial series, boosters – National | 24CA News
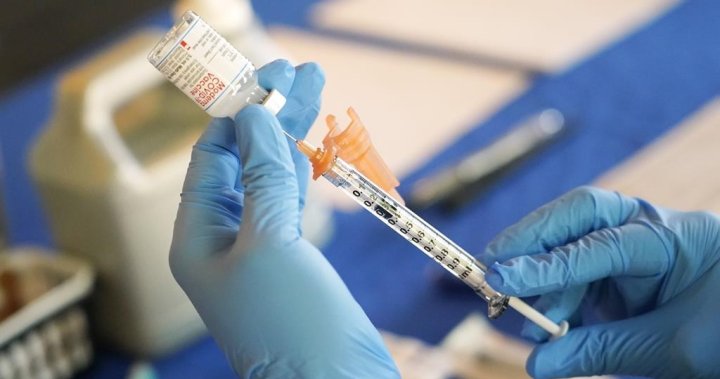
Advisers to the U.S. Food and Drug Administration on Thursday unanimously voted in favor of concentrating on the identical coronavirus pressure for preliminary COVID-19 vaccine doses and boosters going ahead, however some expressed skepticism about whether or not all Americans have to obtain the photographs yearly.
The company is attempting to simplify its COVID-19 vaccine coverage because it considers whether or not to suggest Americans get an annual booster shot for the virus. But a number of members of the skilled advisory group requested for extra strong knowledge on advantages of annual photographs for youthful, more healthy folks.
Read extra:
COVID misinformation led to not less than 2,800 deaths in Canada, $300M in prices: report
Read subsequent:
COVID misinformation led to not less than 2,800 deaths in Canada, $300M in prices: report
“We’re in a very different place. We have a lot of population immunity,” mentioned Hayley Gans, professor of pediatrics at Stanford University Medical Center. “Now that people are immune, how long does that last?”
Vaccine makers Pfizer Inc PFE.N with companion BioNTech SE 22UAy.DE and Moderna Inc MRNA.O launched late final 12 months up to date variations of their COVID vaccines tailor-made to focus on Omicron variants in addition to the unique coronavirus. In the United States, these have been used solely as booster photographs.
The FDA advisory group unanimously backed utilizing these photographs for the first collection for individuals who have but to be vaccinated in opposition to COVID-19 as effectively.
The FDA mentioned it envisioned holding a gathering later within the 12 months to find out the composition of photographs for the autumn, though some vaccine makers would possibly be capable of produce up to date photographs extra shortly.

Pfizer/BioNTech and Moderna have been in a position to produce the presently out there messenger RNA boosters in about three months final 12 months, however Novavax Inc NVAX.O mentioned on Thursday it could require six months to make a brand new model of its protein-based COVID-19 vaccine designed to match circulating coronavirus variants.
FDA would contemplate an earlier timeline for vaccines like Novavax’s following the corporate’s manufacturing evaluation, Peter Marks, director of the company’s Center for Biologics Evaluation & Research, mentioned.
Health officers within the Biden administration have steered that annual, up to date COVID-19 booster photographs might present a excessive stage of safety in opposition to extreme illness.
(Reporting by Leroy Leo in Bengaluru, Michael Erman in Maplewood, New Jersey; Editing by Bill Berkrot)